Classify the Following as Intensive or Extensive Properties of Copper.
Intensive properties of copper are. It is a measure of strength.

Chem 170 Ch 0 Flashcards Quizlet
Start studying Classify each property as intensive or extensive.

. Explain why samples of gold and copper can have the same extensive properties but not the same intensive properties. Classify the following properties of a system as extensive or intensive. Intensive property is regardless of the quantity of the sample.
The mass of iron present in your blood. Classify the following as an intensive or an extensive property. The ratio between two extensive properties is an intensive property.
An intensive property is a physical quantity whose value does not depend on the amount of the substance for which it is measured. Length is an extensive property. A All matter is made of tiny indestructible particles called atoms.
This is the best answer based on feedback and ratings. Extensive properties depend on the amount of matter present for example the mass of gold. Measurable properties fall into one of two categories.
Hardness is an intensive property. Classify the following as intensive or extensive properties of copper. I Mass ii Length iii Melting point iv Volume v Luster.
Drag the relevant items to their respective bins. Has a volume of 894mL. It is a property that measures the amount of matter that an object contains.
The number of calories of energy made available to your body when you consume 10g of sugar. However the weight of the same body on the Moon will be much lower while its mass will remain the same. Or give an example of each substance in the appropriate section of your flowchart provided and tape this into your composition book.
C opper is a tough ductile and malleable material. For example 10 kilograms of solid Copper contains a higher mass than 2 kilograms of the same metal. Which of the following is classified as an extensive property.
The vast property varies according to the quantity of the sample. Extensive properties are properties of the matter which depend on the amount of matter that is present in the system or sample. It is the gravitational force acting on an object.
Extensive properties of copper are. See the answer See the answer done loading. Examples of extensive properties.
An extensive property is a property that depends on the amount of matter in a sample. Volume is an extensive property. Examples of extensive properties.
If the system is divided by a wall that is permeable to heat or to matter the temperature of each subsystem is identical. Color temperature and solubility are examples of intensive properties. An intensive property is a property of matter that depends only on the type of matter in a sample and not on the amount.
Classify the following as an intensive or an extensive property. Do not depend on vary with the amount of the matter present. In Chemistry there are two 2 main types of physical.
According to the definitions density pressure and temperature are intensive properties and volume internal energy are extensive properties. 100 12 ratings lustrous yellow in color - intensive has a. A physical property refer to the properties of an object that can be measured and observed without changing altering the chemical composition of the object.
Classify at least 12 of the following substances to the best of your ability. Intensive properties do not depend on the amount of matter present for example the density of gold. Malleability is an intensive property.
Examples of extensive properties include. Intensive properties are independent of the amount of substance present. Intensive - same for any same-sized sample.
Classify the following properties as intensive or extensive properties of copper. An intensive property is a physical property of a system that does not depend on the system size or the amount of material in the system. Mass and volume are examples of extensive properties.
A i ii iv b ii iii c ii iii v d iii v. For example the temperature of a system in thermal equilibrium is the same as the temperature of any part of it. Classify each of the following as a homogeneous or a.
The word intensive was derived from intensives. Name two categories used to classify properties of matter. Density temperature pressure etc are some examples of intensive properties.
The number of calories of energy you derive from eating a banana. When ammonia gas is reacted with copperII oxide nitrogen gas water vapor and copper metal are. Extensive properties do depend on the amount of matter that is present.
The size of intensive properties does not change. The other key properties exhibited by copper and its alloys include. Or they are bulk properties.
Other intensive properties include color temperature density and solubility. You could cut off the small end sticking out and it would have. On the earths surface the weight of an object is equal to its mass.
These properties make copper extremely suitable for tube forming wire drawing spinning and deep drawing. Intensive - same for any 10g portion of sugar. Learn vocabulary terms and more with flashcards games and other study tools.
Volume pressure energy thermal expansion coefficient and viscosity. An intensive property is a property of matter that depends only on the type of matter in a sample and not on the amount. Classify the following as an intensive or an extensive property.
An extensive property is considered additive for subsystems. The copper wire shown in the picture below has a certain electrical conductivity. Intensive and extensive properties Explain why all samples of a given substance have the same.
Classify each of the following as an observation a law or a theory. For a strict interpretation of the van der Waals equation how does the potential energy of interaction. Extensive - depends on size and sugar content of the banana.
Which of the following is not an example of an intensive property. Density hardness thermal conductivity electrical resitivity etc. Physical properties can be classified as intense properties or extensive properties.
Heat is an example of an extensive property and temperature is an example of an intensive property. 0 K is the lowest temperature that can be attained theoretically. Intensive and Extensive Properties.
Classify the following as intensive or extensive properties of zinc a.

Solved Intensive Properties And Extensive Properties Chegg Com
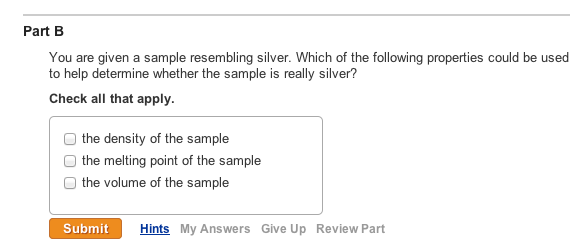
Solved Classify The Following As Intensive Or Extensive Chegg Com
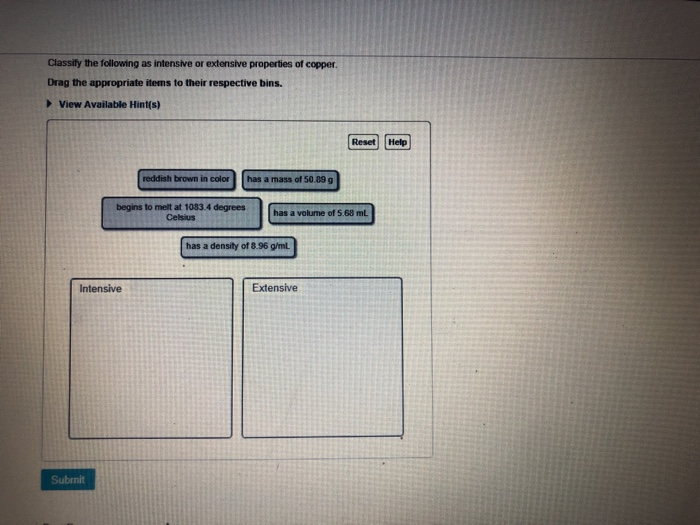
Solved Intensive Properties And Extensive Properties Chegg Com

Solved Intensive Properties And Extensive Properties Chegg Com
Comments
Post a Comment